Abstract
Foodborne illness often occurs by consuming food contaminated by foodborne pathogens (viruses, bacteria or their toxins, parasites, or other biological agents). The most common foodborne diseases are caused by Norovirus, Salmonella, and Staphylococcus aureus. Noroviruses (NoVs) are emerging as one of the foremost enteric pathogens of foodborne disease worldwide. These viral agents are the cause of more than 95% of epidemic non-bacterial gastroenteritis. HBGA-like molecules present in commensal enteric bacteria can interact with Human NoV. This binding can provide a higher resistance of NoV against heat stress treatment. These findings underscore the importance of considering the interaction between bacteria and NoV’s while assessing norovirus-decontamination in the food processes and formulations. This review explores identifying the interactions between Virus-Like Particles (VLPs) of Human Norovirus and bacterial components, focusing on the role of HBGA-like molecules. This study also highlights the potential of identification of bacterial-virus binding by fluorescent labeling. Understanding these interactions can improve detection and intervention strategies, ultimately enhancing food safety and public health outcomes.
Introduction
The strong connection between food consumption and human diseases was recognized early by Hippocrates (460 B.C.), who showed that there is an association between food consumption and human illness (Bintsis and Thomas, 2017). Foodborne illness often occurs by consuming food contaminated by foodborne pathogens (viruses, bacteria or their toxins, parasites, or other biological agents). Foodborne illness occurs when a pathogen is ingested with food and establishes itself by multiplying in the human host, or when a toxigenic pathogen establishes itself by producing a toxin in the food products, which is then ingested by the human host. Thus, foodborne illness is generally classified into two categories: foodborne infection and foodborne intoxication. The most common foodborne diseases are caused by Norovirus, Salmonella, Clostridium perfringens, Campylobacter, and Staphylococcus aureus (Scallan et al., 2011).
Staphylococcal food-borne disease (SFD) is one of the most common foodborne diseases worldwide, resulting from eating food contaminated with the toxins produced by Staphylococcus aureus. S. aureus is a Gram-positive, cocci-shaped bacteria that can cause a wide spectrum of infections (Kadariya et al., 2014). S. aureus is an opportunistic bacterium that does not form spores but can cause the contamination of food products during food preparation and processing. Staphylococcus epidermidis is also a coagulase-negative, gram-positive, coccus, opportunistic bacteria that can cause virulence once it invades the human host. Enterobacter cloacae is a Gram-negative, facultatively anaerobic, rod-shaped bacterium belonging to the family Enterobacteriaceae. E. cloacae has emerged as the second most prevalent pathogen from ready-to-eat food, which can be endemic in food processing environments (Nyenje et al., 2013).
Noroviruses (NoVs), also called the “winter vomiting bug,” are emerging as one of the foremost enteric pathogens of foodborne disease worldwide. These viral agents are the cause of more than 95% of epidemic non-bacterial gastroenteritis, with some lethal cases (Campillay-Véliz et al., 2020). Human noroviruses belong to the family Caliciviridae and the genus Norovirus, having a relatively small, single-stranded, positive-sense, linear RNA genome (7500-7700 nucleotides in length) containing three open reading frames (ORFs) (Chan et al, 2017). ORF1 encodes a polyprotein that is composed of six non-structural proteins (p48, nucleoside-triphosphatase (NTPase), p22, VPg, protease, and the RNA-dependent RNA polymerase); ORF2 encodes the major structural protein of the viral capsid VP1, and ORF3 encodes the minor structural protein of the capsid VP2 (Campillay-Véliz et al., 2020).
Noroviruses are genetically very diverse and divided into different genogroups (from genogroup I [GI] to genogroup X [GX]) (Chhabra et al., 2019) determined by the Viral Protein 1 (VP1), amino acid sequence. However, only GI, GII, and GIV are mainly responsible for acute gastroenteritis in humans. For the last two decades, NoVs belonging to the GII.4 lineage have been found to be responsible for the outbreaks of at least six pandemics (Chan et al., 2015). Remarkably, the novel GII.17 Kawasaki variant emerged as a major outbreak in several East Asian countries, including different areas of the US and Europe, surpassing GII.4 Sydney as the predominant strain in the year 2014/15(Chan et al., 2017). The expression pattern of HBGAs is important in NoV susceptibility since they function as attachment factors/receptors for cellular entry into human gastrointestinal cells that may help in initiating infection (Blazevic et al., 2016). The genotype or strain of each human NoV has its recognition profile of HBGA. For instance, virus-like particles (VLPs) of Norwalk virus (NV/68), GI.1 strain, and the prototype sin of norovirus, bind to HBGAs in saliva from secretor-positive individuals and preferentially bind to H type 1, Lewis b (Leb), and type A carbohydrates (Hutson et al., 2004). HBGA-like molecules are present on some commensal enteric (Enterobacter cloacae) bacterial surfaces, and it has been shown that the HBGAs present in Enterobacter cloacae increase viral infectivity by enhancing the attachment of the NoV to the target cells (Almand et al., 2017). Expanding these results, there is another study that aimed to investigate the role of HBGA-expressing bacteria (Escherichia coli, Enterobacter cloacae ) on the VLPs of human NoV during heat stress treatment as the heat treatment has proven to be the most effective strategy widely used in the food industries (Li et al., 2015). This study suggested that the bacteria expressing HBGA-like moieties may protect the norovirus by aiding resistance to the food processing treatments, resulting in the facilitation of transmission (Li et al., 2015).
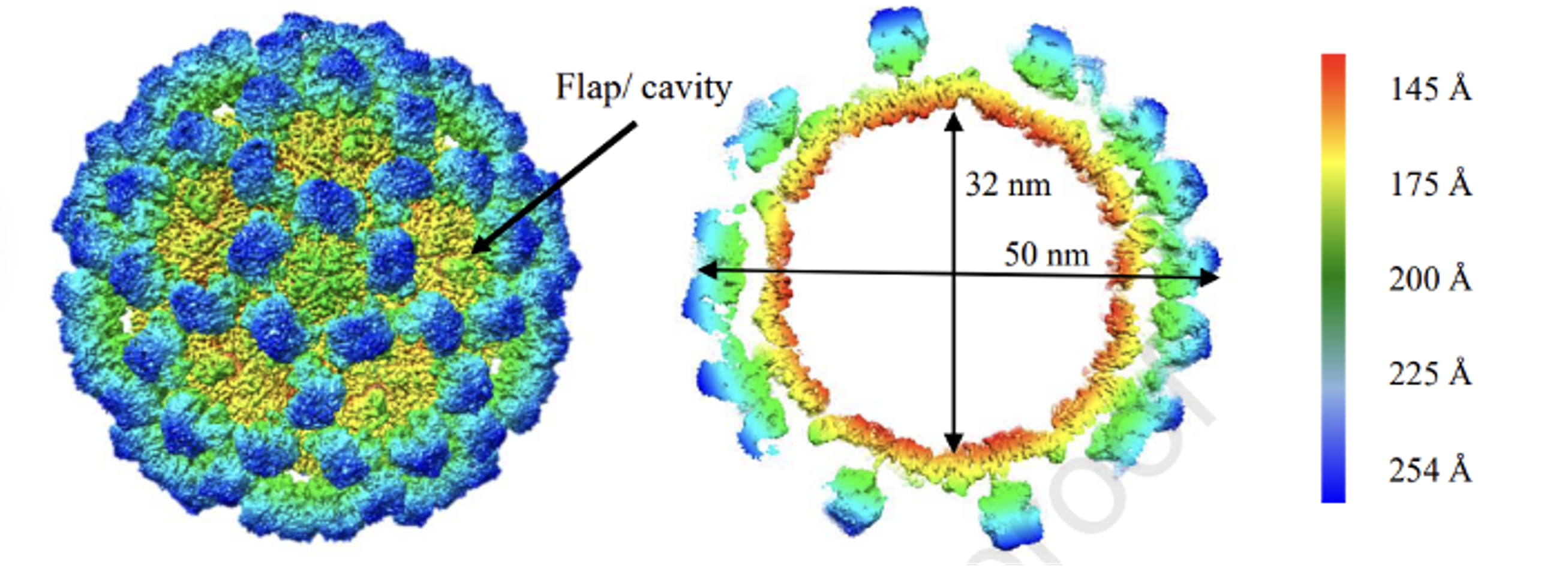
Figure 1: Cryo-EM structure of GII.4c VLPs GII.4c VLPs expressed in insect cells (Ruoff et al., 2019).
Noroviruses are highly resistant to harsh external environments and regarded as non-cultivable as neutralization assay is not yet possible (Belliot et al., 2001), surrogate is the most viable option to use in the lab. The concept of a surrogate is the use of non-pathogenic substitutes that mimic the behavior of pathogenic microorganisms in specific environmental conditions. VLPs, or virus-like particles, are non-infectious particles with the ability to self-assemble, mimicking the form and size of a virus particle but lacking the genetic material so they are not capable of infecting the host cell. In the case of human norovirus, these particles are generated from the expression of the VP1, which self-assembles to create intact viral capsids lacking genetic material (Mohsen and Bachmann, 2022; Nooraei et al., 2021).
The Impact of Enteric Viruses and Biofilms on Foodborne Illness
Enteric viruses (HuNoV, hepatitis A and E viruses, and rotavirus) are the leading cause of foodborne illness outbreaks, posing a big public health burden. Fresh produce, meats, and processed foods provide an ideal transmission route for these viruses. In addition, biofilm is a serious threat in many food processing sectors as it can persist in various types of equipment and non-porous surfaces used in food processing industries (Gagné et al., 2022). This persistence may lead to an increased microbial burden on the food industry by affecting the final product through spoilage or contamination, reducing the shelf life of the product, and resulting in increasing the risks of infectious diseases originating from food sources. Staphylococcus, Streptococcus, Salmonella enteritidis, and Escherichia coli O157:H7 are foodborne pathogens that are mainly responsible for foodborne diseases (Liu et al., 2023). These foodborne pathogens often form biofilms on living and abiotic surfaces, resulting in food spoilage and contamination, hygiene, and safety failure in the food industries.
The Interaction Between Enteric Viruses and Bacteria
In vivo, enteric viruses and commensal bacteria have always coexisted and evolved in the gastrointestinal microbiota. In the study conducted by (Amarasiri and Sano, 2019), norovirus and poliovirus have the ability to attach to the bacterial cell wall components, including EPS (extracellular polymeric substances), lipopolysaccharide and peptidoglycan. Moreover, another study conducted by (Cai et al.,2018) demonstrated that norovirus and rotavirus have the ability to interact with the specific bacterial strain E. cloacae, E. coli Nissle, and some lactic acid bacteria (LAB) (L. rhamnosus, B. bifidum, etc.), can also be found in biofilms in the industries of food processing.
The association between poliovirus, Cryptosporidium parvum, Giardia lamblia, and a multitude of phages with biofilm (Helmi et al., 2008), as well as enteroviruses and noroviruses associated with biofilms from wastewater tanks (Skraber et al., 2009) were observed. Also, another recent study investigated the viral compositions of biofilms from wastewater treatment plants (Petrovich et al., 2019). These studies indicate that the association between enteric viruses and bacteria may play a potential role in promoting easier entry of viral particles into the biofilm. Another study reported that the biofilms formed on surfaces used in the Agro-food sector with spoilage bacteria and Lactic Acid Bacteria (LAB) may promote the adhesion of infectious foodborne viruses under specific conditions and, the three viruses (Hepatitis A, Rotavirus and Murine Norovirus) used in this research showed that neither have the same behavior nor the same binding abilities under same experimental conditions (Gagné et al., 2022).
Facilitation of Viral Infection by Bacteria and/or Biofilm
Murine Norovirus (MNV) has the ability to interact with Pseudomonas aeruginosa biofilms where the biofilms either entrap the particles of MNV-1 or MNV-1 particles can infiltrate mature biofilms (Soorneedi and Moore, 2022). This study demonstrated that the binding between norovirus and bacteria may induce resistance to the inactivation process such as heat, ultraviolet light or chlorine inactivation due to the surface masking properties of the biofilm. The bacterial cellular components peptidoglycan (PEP), and lipopolysaccharide LPS showed influence on Tulane Virus (TV), a member of the Caliciviridae family and cultivable norovirus surrogate (Soorneedi and Moore, 2022). Precisely, Escherichia coli O111:B4 LPS and Bacillus subtilis PEP were found to show recalcitrance to TV for both thermal and chlorine inactivation. The commensal bacteria were reported to show pathogenicity with other enteric viruses as well as its surrogate virus (MNV) (Soorneedi and Moore, 2022). The direct interaction between the virus and bacteria plays a potential role in viral infectivity which highlights the importance of considering the interaction of bacteria and biofilms while evaluating norovirus-decontamination food processes and formulations.
Challenges in Detecting Bacteria-VLP Binding
Detection of Bacteria-VLP (Virus-like Particle) binding can not only be very tricky but also time-consuming. First of all, both bacteria and viruses are small. Bacteria are in the micrometer (Escherichia coli: 1.5 µm long and 0.5 µm wide) range whereas most viruses are submicroscopic, (non-enveloped Norovirus virion: approximately 27 nm (Shiomi et al., 2009; Chan et al., 2017). Without the use of very sophisticated and specialized tools such as electron microscopy, atomic force microscopy or fluorescence-based imaging it is not possible to visualize the two entities. In addition to size, the nature of their dynamics, heterogeneity, sample complexity, and background noise make it very difficult to detect bacteria-VLP interaction (Nooraei et al., 2021; Roldão et al., 2017).
Advancements in Microbial Identification Methods
Fast detection and identification of microorganisms have always been an important feature from medicine to industry. During the last decade, scientists have raced to develop more prompt and effective means of microbial identification. Phenotypic classification was the only identification approach for many years despite its uncertainties and inaccuracy in results (Franco-Duarte et al., 2019).With more advancements in microbiological research, more sophisticated methods emerged. Such as mass spectrometry-based methods, spectroscopic methods, electrokinetic separation methods, molecular methods etc (Franco-Duarte et al., 2019). These new methods aided in the fast and accurate detection of microorganisms but these methods have their own flaws. For example, Fourier transform infrared spectroscopy (FTIR) is a versatile, fast, non-invasive, easy-to-perform analytical technique that is a chemical and label-free procedure which gives a clear elucidation about the chemical composition and the physical state of the entire sample where several biomolecules can be analyzed. An economic biochemical characterization of complex biological systems, comprising intact cells, tissues, and even whole-model organisms can be obtained by FTIR (Franco-Duarte et al., 2019). However, this technique requires extensive multivariate statistical analysis in order to extract only the relevant information about the biological process under study. Molecular techniques such as 16S rRNA PCR-Sequencing and real-time PCR (RT-PCR) are very effective for the detection of bacteria. However, these techniques can be very expensive and time-consuming. Another drawback of molecular techniques is the detection of bacteria-VLPs conjugate. As VLPs are derived from genetic materials, detection of just VLPs or bacteria-VLPs conjugate is not possible by techniques that involve sequencing (Mohsen and Bachmann, 2022; Nooraei et al., 2021).
Fluorescent-Based Techniques
In recent years, Fluorescent-based labeling techniques have proven to be a powerful tool for the identification of both bacteria and viruses. A study by Ji et al. (2022) emphasized that the development of fluorescent-based dyes to detect bacteria has received significant attention in the past few decades. The study also mentioned that fluorescent probes can be targeted based on different target sites of bacteria (Ji et al., 2022). According to Yoon et al. (2021), fluorescence probes can detect bacteria with “off-on” fluorescence change based on bacterial-specific reactions and interactions, enabling real-time imaging and quantitative analysis of bacteria in vitro and in vivo. In addition, the validation of the probes was demonstrated by using a variety of biological models such as gram-negative and positive bacteria, antibiotic-resistant bacteria, infected cancer cells, tumor-bearing, and infected mice. Additionally, the working principles that were used (internal charge transfer (ICT), twisted intramolecular charge transfer (TICT) and aggregation-induced emission (AIE)) have also been discussed (Yoon et al., 2021). Fluorescent-based techniques have also been employed to detect and track viruses.

Figure 2: Fluorescent labeling strategies for different viral components.
Fluorescence-based imaging techniques can uncover the life cycle of viruses including virus adsorption, internalization, and transportation in real-time, providing a sophisticated spatiotemporal dynamic process and mechanism of virus infection (Liu et al., 2023). For successful virus tracking, the selection of appropriate fluorescent labels and virus-labeling compounds is crucial. According to Liu et al. (2023), various types of fluorescent labels, such as organic dyes, quantum dots, and fluorescent proteins, can be used to label viruses. Self labeled fusion tags is a distinct example which has the ability to supply fluorescent ligands that specifically bind to the target protein in the living cells. During the event of protein expression in live cells, these specific fluorescent ligands are added for labeling of proteins. Another benefit of the self-labeled fusion peptides is short sequence, making it easier for the creation of various fluorescent ligands for specific use (Liu et al., 2023). With the development of virus-tracking technology, valuable insights in the dynamic nature of viral infection can be obtained (Liu et al., 2023). Problem arises when the detection of bacteria-virus conjugate has to be taken into account. It is important to efficiently detect and identify bacteria-virus conjugate as direct binding between viruses and the host microbiome is responsible for altering infection for many of the viruses (Madrigal and Jones, 2020). They also observed that commensal bacteria enhance the human norovirus B cell infection rate. Although the conjugate of bacteria and viruses can be detected by real-time PCR (RT-PCR) that can be time-consuming and expensive (Mohsen and Bachmann, 2022). In addition, high-tear virus stock and in vitro cultivation techniques are required for the quantification of viruses in bacteria-virus conjugate. Additionally, requirement of purification steps prior to PCR and quantification of amplified samples after PCR. This adds further time and expense into the entire process. Furthermore, detection of VLPs is also not possible by sequencing as VLPs do not contain any genetic material.
Challenges of Antibody-Based Flow Cytometry in the Detection of Bacteria-VLP Conjugate
In many studies, antibody-based flow cytometry has been used to identify the bacterial-VLPs conjugate (Carlson-Jones et al., 2016; Madrigal and Jones, 2020). However, the accuracy and the detection level were low (Madrigal and Jones, 2020). Flow cytometry is an effective technique for direct detection and quantification of bacteria in sterile biological fluids; however, the low volume of the sample, sample viscosity, and cell concentration can impair the count of particles (Rubio et al., 2019).
ELISA in Bacteria-VLP Conjugate Detection: Strengths and Limitations
Another technique that is often utilized for bacteria-VLPs conjugate detection is ELISA (enzyme-linked immunosorbent assay). ELISA is also a reliable technique that is used for the detection of small scale VLPs production as well (Estienney et al., 2022). This technique is highly sensitive, reliable, provides quantitative analysis and is cost-effective compared to other detection techniques that are currently available (Sakamoto et al., 2017). However, this method has its own limitations. Firstly, optimization of various parameters such as antibody selection, and coating conditions are required for the detection of specific bacteria-VLPs conjugate. Secondly, the dynamic range is low which may lead to inaccurate quantification of bacteria-VLPs conjugate. In addition, real-time monitoring of bacteria-VLP conjugate binding events cannot be monitored by ELISA as it provides endpoint measurement (Sakamoto et al., 2017). This can also lead to cross-reactivity of the antibody with other molecules that are present in the sample, generating false positive results (Sakamoto et al., 2017). Furthermore, ELISA can be time-consuming where each test run might take several hours including incubation and washing steps.
Alexa Fluor Dyes for Sensitive Detection
One possible solution can be the utilization of Alexa Fluor dyes as fluorescent probes. In comparison to conventional dyes, Alexa Fluor dyes exhibit better photo-stability and superior brightness (Tu et al., 2006). These characteristics help to obtain optimal signal-to-noise ratios and longer imaging durations. In addition, the dye is available in varieties of spectrum including visible light which aids in selection of appropriate dye for specific application. Additionally, Alexa Fluor-conjugated antibodies or other biomolecules can be designed to specifically target bacteria or VLPs, enabling sensitive and specific detection of the conjugates (Tu et al., 2006). Another advantage of using Alexa Fluor dyes is it can effectively function in lower conjugate concentration reducing both the cost and chances of non-specific binding (Tu et al., 2006). The scientific papers that have been demonstrated show the significance of fluorescent-based labeling techniques for identification and detection of bacteria-VLPs binding events. Techniques such as flow cytometry, ELISA, western blotting also offer a way to detect bacteria-VLPs conjugate but these techniques are hindered by long preparation time, high volume of conjugate and background noise for conjugate detection. Fluorescence-based techniques offer high sensitivity, real-time tracking, and quantitative analysis, providing valuable insights into the interaction between bacteria and VLPs. For example, use of Alexa Fluor dyes as fluorescent probes can increase both specificity and detection rate of bacteria-VLPs conjugate and low concentration. However, this method also suffers from background noise in high concentration of bacteria-VLPs conjugate, limited dynamic range and intensive optimization in assay development (Liu et al., 2020).To ensure accurate and reliable results, careful optimization and validation in experimental protocol are necessary.
References:
- Almand, A., Moore, M.D. and Jaykus, L.A. (2017) ‘Virus-Bacteria interactions: an emerging topic in human infection,’ Viruses, 9(3), p. 58. https://doi.org/10.3390/v9030058.
- Amarasiri, and Sano, D. (2019) ‘Specific Interactions between Human Norovirus and Environmental Matrices: Effects on the Virus Ecology,’ Viruses, 11(3), p. 224. https://doi.org/10.3390/v11030224.
- Belliot, et al. (2001) ‘Characterization of capsid genes, expressed in the baculovirus system, of three new genetically distinct strains of “Norwalk-Like viruses”,’ Journal of Clinical Microbiology, 39(12), pp. 4288–4295. https://doi.org/10.1128/jcm.39.12.4288-4295.2001.
- Bintsis, and Author_Id, N. (2017) ‘Foodborne pathogens,’ AIMS Microbiology, 3(3), 529–563. https://doi.org/10.3934/microbiol.2017.3.529.
- Blazevic, et al. (2016) ‘Development and maturation of norovirus antibodies in childhood,’ Microbes and Infection, 18(4), pp. 263–269. https://doi.org/10.1004016/j.micinf.2015.12..
- Cai, et al. (2018) ‘Response of Formed‐Biofilm of Enterobacter cloacae, Klebsiella oxytoca, and Citrobacter freundii to Chlorite‐Based Disinfectants,’ Journal of Food Science, 83(5), pp. 1326–1332. https://doi.org/10.1111/1750-3841.14149.
- Campillay-Véliz, P. et al. (2020) ‘Human norovirus proteins: implications in the replicative cycle, pathogenesis, and the host immune response,’ Frontiers in Immunology,11. https://doi.org/10.3389/fimmu.2020.00961.
- Carlson-Jones, J. a. P. et al. (2016) ‘Enumerating Virus-Like particles and bacterial populations in the sinuses of chronic rhinosinusitis patients using flow cytometry,’ PLOS ONE, 11(5), p. e0155003. https://doi.org/10.1371/journal.pone.0155003.
- Chan, C.W. et al. (2015) ‘Rapid emergence and predominance of a broadly recognizing and fast-evolving norovirus GII.17 variant in late 2014,’ Nature Communications, 6(1).https://doi.org/10.1038/ncomms10061.
- Chan, C.W., Kwan, H.S. and Chan, P.K.S. (2017) ‘Structure and genotypes of noroviruses,’ in Elsevier eBooks, pp. 51–63.https://doi.org/10.1016/b978-0-12-804177-2.00004-x.
- Chan, C.W. et al. (2017) ‘Global Spread of Norovirus GII.17 Kawasaki 308, 2014–2016,’ Emerging Infectious Diseases, 23(8), pp. 1359–1354.https://doi.org/10.3201/eid2308.161138.
- Chhabra, et al. (2019) ‘Updated classification of norovirus genogroups and genotypes,’Journal of General Virology,100(10),pp.1393–1406.https://doi.org/10.1099/jgv.0.001318.
- Estienney, et al. (2022) ‘Epidemiological impact of GII.17 human noroviruses associated with attachment to enterocytes,’ Frontiers in Microbiology, 13.https://doi.org/10.3389/fmicb.2022.858245.
- Feng, J. et al. (2014) ‘An Optimized SYBR Green I/PI Assay for Rapid Viability Assessment and Antibiotic Susceptibility Testing for Borrelia burgdorferi,’ PLOS ONE, 9(11), p. e111809. https://doi.org/10.1371/journal.pone.0111809.
- Franco-Duarte, et al. (2019) ‘Advances in chemical and biological methods to identify Microorganisms—From Past to present,’ Microorganisms, 7(5), p. 130.https://doi.org/10.3390/microorganisms7050130.
- Gagné, -J., Savard, T. and Brassard, J. (2022) ‘Interactions between infectious foodborne viruses and bacterial biofilms formed on different food contact surfaces,’ Food and Environmental Virology, 14(3), pp. 267–279.https://doi.org/10.1007/s12560-022-09534-z.
- Helmi, K. et al. (2008) ‘Interactions of Cryptosporidium parvum , Giardia lamblia , Vaccinal Poliovirus Type 1, and Bacteriophages φX174 and MS2 with a Drinking Water Biofilm and a Wastewater Biofilm,’ Applied and Environmental Microbiology, 74(7), 2079–2088. https://doi.org/10.1128/aem.02495-07.
- Hutson, M., Atmar, R.L. and Estes, M.K. (2004) ‘Norovirus disease: changing epidemiology and host susceptibility factors,’ Trends in Microbiology, 12(6), pp. 279–287. https://doi.org/10.1016/j.tim.2004.04.005.
- Ji, et al. (2022) ‘Recent Progress in Identifying Bacteria with Fluorescent Probes,’Molecules, 27(19), p. 6440. https://doi.org/10.3390/molecules27196440.
- Kadariya, J., Smith, T.C. and Thapaliya, D. (2014) ‘Staphylococcus aureusand Staphylococcal Food-Borne Disease: An Ongoing Challenge in Public Health,’ BioMed Research International, 2014, pp. 1–9. https://doi.org/10.1155/2014/827965.
- Li, D. et al. (2015) ‘Binding to histo-blood group antigen-expressing bacteria protects human norovirus from acute heat stress,’ Frontiers in Microbiology, 6. https://doi.org/10.3389/fmicb.2015.00659.
- Liu, D., Pan, L., Zhai, H., Qiu, H., & Sun, Y. (2023). Virus tracking technologies and their applications in viral life cycle: research advances and future perspectives. Frontiers in Immunology, 14. https://doi.org/10.3389/fimmu.2023.1204730
- Liu, S. et al. (2020) ‘Single-Virus tracking: From imaging methodologies to virological applications,’ Chemical Reviews, 120(3), 1936–1979. https://doi.org/10.1021/acs.chemrev.9b00692.
- Liu, et al. (2023) ‘Biofilm formation and control of foodborne pathogenic bacteria,’Molecules, 28(6), p. 2432. https://doi.org/10.3390/molecules28062432.
- Madrigal, J.L. and Jones, M.K. (2020) ‘Quantifying human norovirus virus-like particles binding to commensal bacteria using flow cytometry,’ Journal of Visualized Experiments [Preprint], (158). https://doi.org/10.3791/61048.
- Mohsen, M.O. and Bachmann, M.F. (2022) ‘Virus-like particle vaccinology, from bench to bedside,’ Cellular & Molecular Immunology, 19(9), pp. 993–1011. https://doi.org/10.1038/s41423-022-00897-8
- Naresh, V., & Lee, J. Y. (2021). A review on biosensors and recent development of Nanostructured Materials-Enabled biosensors. Sensors, 21(4), 1109. https://doi.org/10.3390/s21041109
- Nyenje, M.E., Green, E. and Ndip, R.N. (2013) ‘Evaluation of the Effect of Different Growth Media and Temperature on the Suitability of Biofilm Formation by Enterobacter cloacae Strains Isolated from Food Samples in South Africa,’ Molecules, 18(8), pp. 9582–9593. https://doi.org/10.3390/molecules18089582.
- Nooraei, S. et (2021) ‘Virus-like particles: preparation, immunogenicity and their roles as nanovaccines and drug nanocarriers,’ Journal of Nanobiotechnology, 19(1). https://doi.org/10.1186/s12951-021-00806-7.
- Petrovich, M. et al. (2019) ‘Viral composition and context in metagenomes from biofilm and suspended growth municipal wastewater treatment plants,’ Microbial Biotechnology, 12(6), pp. 1324–1336. https://doi.org/10.1111/1751-7915.13464.
- Roldão, A. et al. (2017) ‘Viruses and Virus-Like Particles in Biotechnology: Fundamentals and Applications,’ in Elsevier eBooks, pp. 633–656. https://doi.org/10.1016/b978-0-12-809633-8.09046-4.
- Rubio, E. et al. (2019) ‘Evaluation of flow cytometry for the detection of bacteria in biological fluids,’ PLOS ONE, 14(8), e0220307. https://doi.org/10.1371/journal.pone.0220307.
- Ruoff, et al. (2019) ‘Structural basis of nanobodies targeting the prototype norovirus,’Journal of Virology, 93(6). https://doi.org/10.1128/jvi.02005-18.
- Sakamoto, S. et al. (2017) ‘Enzyme-linked immunosorbent assay for the quantitative/qualitative analysis of plant secondary metabolites,’ Journal of Natural Medicines, 72(1), pp. 32–42. https://doi.org/10.1007/s11418-017-1144-z.
- Scallan, E. et al. (2011) ‘Foodborne illness acquired in the United States—Major pathogens,’ Emerging Infectious Diseases, 17(1), pp. 7–15. https://doi.org/10.3201/eid1701.p11101.
- Shiomi, D., Mori, H. and Niki, H. (2009) ‘Genetic mechanism regulating bacterial cell shape and metabolism,’ Communicative & Integrative Biology, 2(3), pp. 219–220. https://doi.org/10.4161/cib.2.3.7930.
- Skraber, et al. (2009) ‘Occurrence and persistence of enteroviruses, noroviruses and F-specific RNA phages in natural wastewater biofilms,’ Water Research, 43(19), pp. 4780–4789. https://doi.org/10.1016/j.watres.2009.05.020.
- Soorneedi, A. and Moore, M.D. (2022) ‘Recent developments in norovirus interactions with bacteria,’ Current Opinion in Food Science, 48, p. 100926. https://doi.org/10.1016/j.cofs.2022.100926.
- Tu, S. et al. (2006) ‘Fiber-optic biosensor employing Alexa-Fluor conjugated antibodies for detection of Escherichia coli O157:H7 and Shiga-like toxins,’ Proceedings of SPIE [Preprint]. https://doi.org/10.1117/12.686316.
- Yoon, A. et al. (2021) ‘Strategies of detecting bacteria using Fluorescence-Based dyes, Frontiers in Chemistry, 9. https://doi.org/10.3389/fchem.2021.743923.
- Zheng, L. et al. (2016) ‘His-tag based in situ labeling of progeny viruses for real-time single virus tracking in living cells,’ Nanoscale, 8(44), pp. 18635–18639. https://doi.org/10.1039/c6nr05806j.
If you like this article, you can go through our other top articles
- A Comprehensive Review of Thalassemia – https://learnlifescience.org/a-comprehensive-review-of-thalassemia/
- DNA Sequencing Methods – https://learnlifescience.org/dna-sequencing-methods/
- Biosensors as a Disease Diagnostic Tool: A Comprehensive Review – https://learnlifescience.org/biosensors-as-a-disease-diagnostic-tool-a-comprehensive-review/
- An Overview of Bacterial Genomic DNA Isolation – https://learnlifescience.org/an-overview-of-bacterial-genomic-dna-isolation/
“Since the article has been written to reflect the actual views and capabilities of the author(s), they are not revised for content and only lightly edited to be confirmed with the Learn life sciences style guidelines.”








2 Responses to “Identification of Bacterial-Virus Binding by Fluorescent Labeling”